Time:2018-12-12 Source:Voice of the Chinese Academy of Sciences
Boron, symbol B, is an important nonmetallic element in the periodic table of the second period, the third major group. Due to its strong affinity for oxygen, boron does not occur in nature in its free state, but always exists as a compound. In 1808, the British chemist David and the French chemist Guy Gay-Lussac and Tyner respectively obtained boron, but the purity they obtained was not high, about 60%, until 1909, the American chemist Weintraub produced pure boron.
Boron is a typical lithophile element, which exists in all kinds of rocks of different origin and types. From the perspective of geotectonics, the boron deposits in the world are mainly concentrated in the Pacific and Mediterranean tectonic belts, and the boron resources in the world are mainly distributed in Turkey, the United States, Russia, Chile and China.
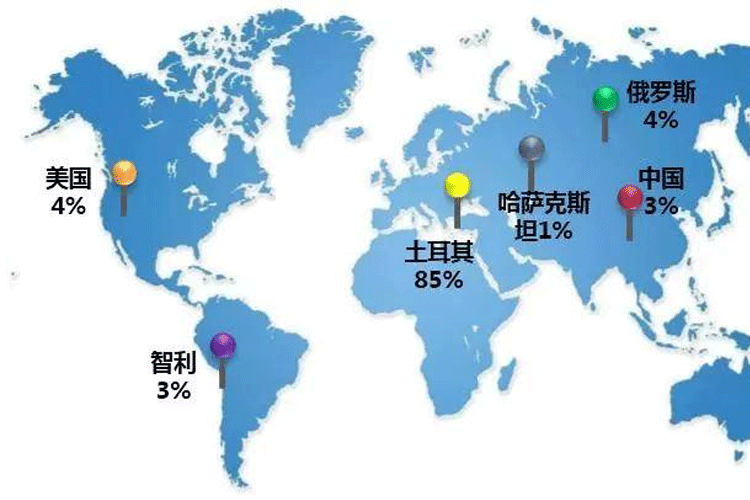
Figure 1 Global distribution of boron resources
Depending on their chemical composition, boron minerals can be divided into three categories: borosilicate minerals, boroaluminosilicate minerals, and borate minerals of alkali and alkaline earth metals. The borosilicate minerals are mainly silica-calborite and cyxanthite. Boro-aluminum silicate minerals are mainly tourmaline and axetstone, but the above two types of boron ore are not suitable for industrial mining, the main industrial development and utilization of alkali metal and alkali earth metal borate minerals, referred to as borate minerals. There are more than 100 kinds of borate minerals, but there are only more than 10 kinds that can be used as industrial boron resources, among which the typical ones are natural borax, anisite, boracite, hard borocalcium, natural boric acid, sodium-calcite and columnium-magnesite.

FIG. 2 Classification of boron ores
Boron has been used for a long time. As early as 200 BC, there was a record that borax was used in the welding of gold in ancient Egypt. Li Shizhen of the Ming Dynasty clearly recorded in the Compendium of Materia Medica that borax had the effect of reducing inflammation and removing blood stasis. Nowadays, boron and borides are an important chemical raw materials, widely used in building materials, light industry, metallurgy, machinery, medicine, agriculture, nuclear industry and other industries. At present, more than 300 boron applications have been found, among which glass, ceramics and detergent are the most important uses, accounting for about three quarters of the world's boron consumption.
Borate and boride are important components of enamel, ceramics and glass. Compared with glass ceramics without boride, boride containing glass has better heat resistance, wear resistance and corrosion resistance, and significantly improved gloss. Borosilicate glass has been recognized by all walks of life in the world for its good performance, and is widely used in solar energy, chemical and pharmaceutical packaging industries.

FIG. 3 Application of boron
Boron is an essential element unique to higher plants. It can bind with free sugars, make sugars easily cross the plasma membrane, and promote the transport of sugars. It has an important effect on the reproductive process of plants, and is closely related to pollen formation, pollen tube germination and fertilization. Boron supply is sufficient, the seeds are full, the root system is good; However, the symptoms of boron deficiency vary greatly in plants. The common characteristics of boron deficiency are flower underdevelopment, underdeveloped root system, and death of growth point, resulting in decreased product and yield, and even no grain harvest.

FIG. 5 Boron deficient crops (photo from Web)
In fact, boron is necessary not only for higher plants, but also for higher animals. Although the physiological function of boron has not been determined, studies have shown that boron has a significant impact on the metabolism, bone development, brain function and immune function of animals. The human body also contains trace amounts of boron, which plays a subtle role in anti-osteoporosis, anti-inflammation, anti-tumor, lowering blood lipid and other aspects. However, if the human body is exposed to a large amount of boron containing substances for a long time, it will cause poisoning.
Due to the special properties of boron and boron compounds, such as light weight, flame retardant, heat resistance, high hardness, high strength, wear resistance and catalytic properties, they play an important role in modern science and technology. They have been on the stage of modern industry, in the national economic sector has a wide range of applications. Boron is found naturally in two stable isotopes. One of them, 10B, has particular uses in the nuclear industry, for controlling and reacting speeds, and as a protective material for nuclear reactors. In recent years, the research of boron containing materials such as boric acid for nuclear use has become a hot research direction.
Based on the diversity of borate structure, there are more and more reports on the synthesis and structural characteristics of various inorganic boron compounds, organic boron compounds, boron halides and refractory boron compounds, which brings the research hotspot of borate application. In the future, the application field of borate will be more and more extensive, and the multipurpose performance of boron will be more and more excellent.
